-
Biopharma Value-Based Pricing: How to Make It Work?
Value-based pricing/contracts (VBCs) have emerged as a way for biopharmaceutical companies and payers to demonstrate how to deliver better health care, while reducing costs and financial risks.
Pressure is mounting in the United States and Europe to decrease overall health care spending and increase stakeholder accountability. For example, the US Department of Health and Human Services aims to tie 90% of Medicare payments to value by 2018. VBC schemes are also common in Italy, Germany, and Spain, and gaining popularity in France and the UK.
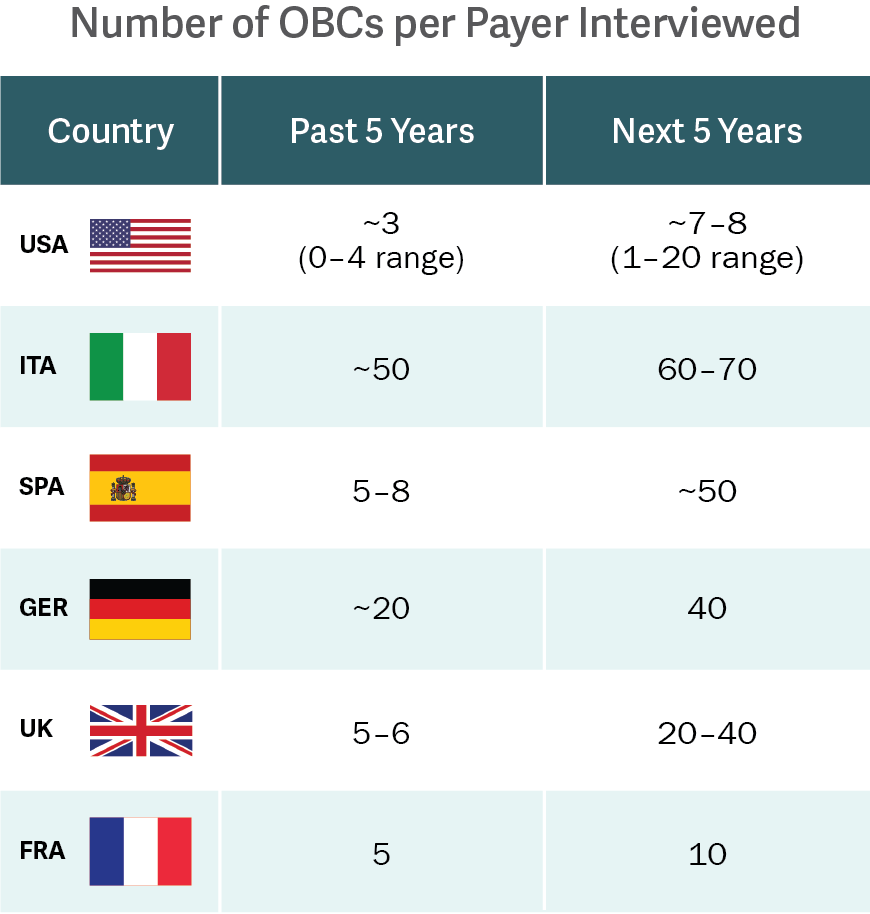
Source: Analysis Group interviews.
In this context, outcomes-based contracts (OBCs) are leading the charge. OBCs are most often confidential agreements that tie price to specified patient outcomes. OBCs can be complex; still, OBCs have been used in many recent high-profile biopharmaceutical product launches, including therapies related to hepatitis, high cholesterol, heart failure, and even oncology.
A recent study, led by Analysis Group and funded by Novartis Pharmaceuticals, is the first to characterize historical OBC activity and trends in the US and EU-5 (France, Germany, Italy, Spain, and the UK) – going beyond the limited information provided by companies’ publicized activity. The research revealed that the level of OBC activity was previously underestimated, and that the US and EU-5 can expect OBC activity to nearly double in the next five years. (See figure.)
Making It Work
From our work supporting the exploration and implementation of VBCs, and OBCs in particular, we can identify several key success factors. First, it is important to recognize that not all payers are the same, in either the US or Europe. To develop a successful VBC, manufacturers must identify each target payer’s motivation. By aligning the contract’s key features with the core rationale for using or not using the product, the benefits become clear to both sides.
Second, we have found that it is best to keep the process as simple as possible. The complexity of designing and implementing a VBC is one of the main hurdles for both manufacturers and payers, so it is helpful to work with an experienced third-party team that can advise on how to keep complexity at bay, while keeping the focus on the value of the product and the deal for both parties. The team should help define and measure the right outcomes with the most relevant data, advise on manufacturer-payer commercial negotiations, and address legal considerations (e.g., anti-kickback statutes, off-label promotion, Medicaid best price).
Thinking through these issues prior to reaching out to payers is often the key to the successful use of VBCs. ■
Eric Wu, Managing Principal
For more information, see “Outcomes-based contracting experience: research findings from U.S. and European stakeholders,” by a team of researchers including Christian Frois and Eric Wu, published in Journal of Managed Care & Specialty Pharmacy, October 2017.

