-
Viewing Recent Opioid Regulations in Context
In recent years, government agencies have grappled with the twin objectives of maintaining access to prescription opioids for those with a legitimate medical need while restricting inappropriate access.
In the past decade, acute and chronic pain patients have benefited from innovation in opioid medicines, including new drug approvals and improved and longer-acting and abuse-deterrent formulations. During this period, opioid prescriptions have increased by more than 25 percent.1 However, opioid-related deaths tripled between 2000 and 2015,2 and the annual economic burden of opioid abuse is a staggering $78.5 billion.3
Consequently, state and federal agencies have been increasing efforts to curb the misuse and abuse of prescription opioids. In 2016, for example, the Centers for Disease Control and Prevention (CDC) published a new guideline recommending shorter durations for opioid prescriptions for chronic pain, and the FDA announced a “black box” warning for immediate-release opioids.
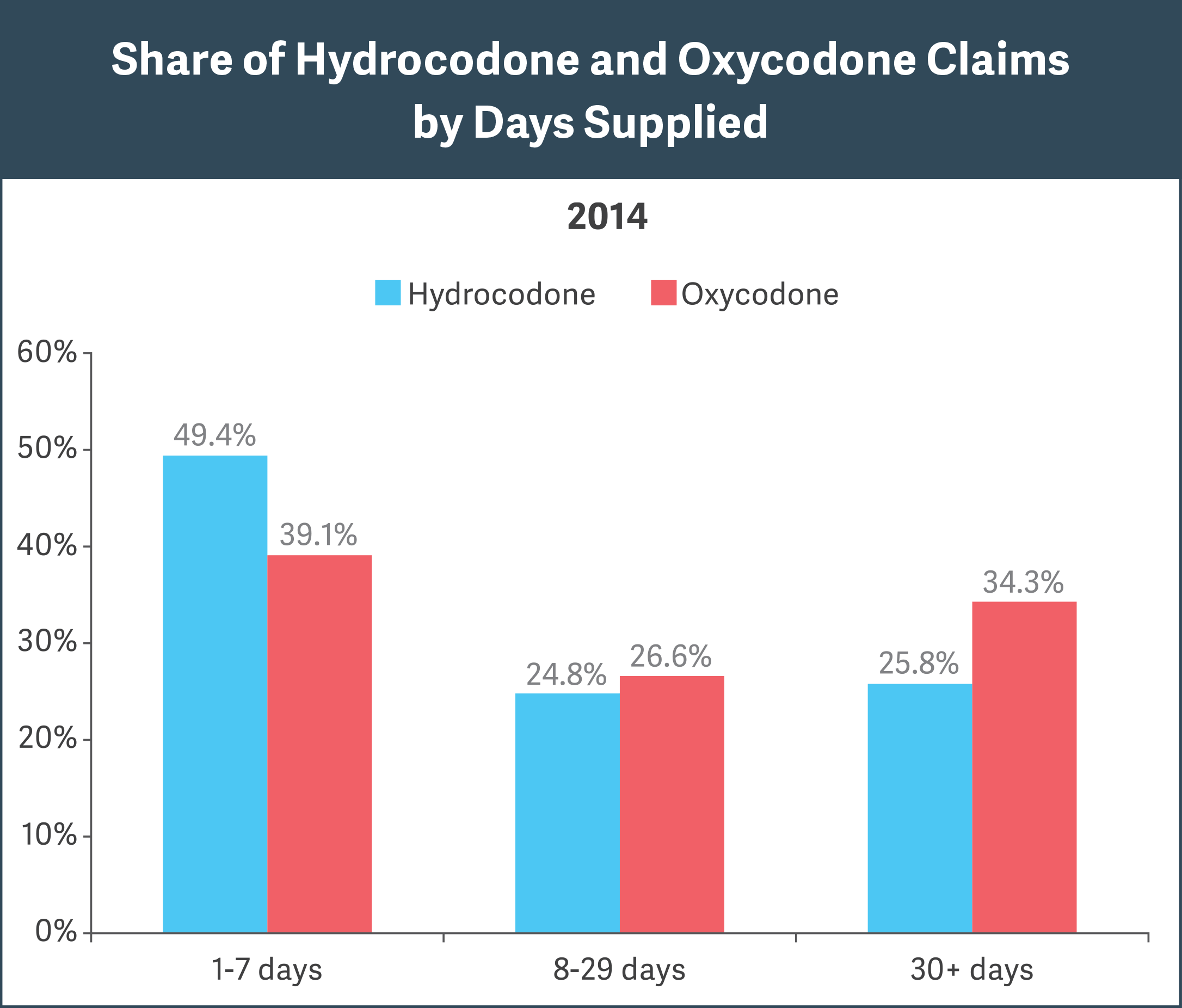
Notes: Hydrocodone/oxycodone claims were identified based on NDCs within the opioid class of drugs. GPI code 65xxx. Analysis excludes drug claims for beneficiaries younger than 18 years. Source: OptumHealth Reporting and Insights database
To put the CDC guideline in the context of existing treatment patterns, Analysis Group analyzed a large set of medical claims data for privately insured patients, with a focus on the past 10 years of oxycodone and hydrocodone prescriptions. Our analysis yielded three main conclusions:
- Adopting the more stringent CDC guideline will likely result in a significant reduction in average days supplied per prescription. The CDC advises that, for acute pain, more than a 7-day supply of opioids “will rarely be needed.” Our analysis found that 50 to 60 percent of oxycodone/hydrocodone prescriptions exceed the 7-day recommendation, with 25 to 35 percent having a 30-day supply. (See figure.)
- The guideline recommends limiting the duration of treatment for chronic pain and reevaluating the need for continued treatment at least every three months. With more than 20 percent of oxycodone/hydrocodone patients in our data having three or more claims annually, time will tell whether the guideline results in a reduction in the proportion of long-term patients.
- While the CDC guidelines do not explicitly address pills per prescription (other than to note that the lowest effective dose is optimal), this is an important consideration for diversion. In our analysis, 35 percent of oxycodone prescriptions and 25 percent of hydrocodone prescriptions exceed 90 pills, with 80 percent of patients receiving three or more pills per day.
Given recent regulatory changes, more research concerning the impact of these changes on abuse/overdose and adequacy of care for patients with legitimate medical need is important. ■Sources:
1Substance Abuse and Mental Health Services Administration, Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health, Sept. 2015
2CDC Morbidity and Mortality Weekly Report, Increases in Drug and Opioid Overdose Deaths — United States, 2000-2014, January 1, 2016/64(50); 1378-82
3As of 2013. Curtis S. Florence, Chao Zhou, Feijun Luo, Likang Xu. “The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States,” 2013. Medical Care, 2016; 54 (10): 901 DOI: 097/MLR.0000000000000625
Crystal Pike, Managing Principal
Kenneth Weinstein, Managing Principal
Pavel Darling, Managing Principal
Paul E. Greenberg, Managing PrincipalAdapted from “Viewing Recent Opioid Regulations In Context,” by Crystal Pike, Kenneth Weinstein, Pavel Darling, and Paul E. Greenberg, published on Law360.com, April 1, 2016.

