-
The Myth of “Price Disconnects” in US Pharma Markets
In pharmaceutical markets, the strategy of introducing a “new and improved” version of an existing brand product is sometimes alleged to be an antitrust violation.
Critics dispute the medical improvements of the new product, claiming that it merely interferes with competition from cheaper generic drugs which are not automatically substitutable.
Some antitrust analysts argue that a “price disconnect” makes pharmaceutical markets particularly vulnerable to this allegedly anticompetitive behavior. They claim that prescribers, patients, insurers, and pharmaceutical benefit managers (PBMs) all fail to make the trade-off between price and quality that is routine in other markets. According to this argument, physicians prescribe drugs with little thought to cost, while patients only track their own costs (co-pays, deductibles, etc.) knowing their insurer will pay the rest.
This argument ignores the economic incentive insurance companies and PBMs have to monitor price/quality trade-offs. These stakeholders regularly employ drug formularies to direct coverage to cost-effective treatments. Pharmacy and therapeutic committees consisting of physicians, pharmacists, nurses, administrators, and quality assurance directors, among others, review and update these formularies, and can adjust the benefit design accordingly.
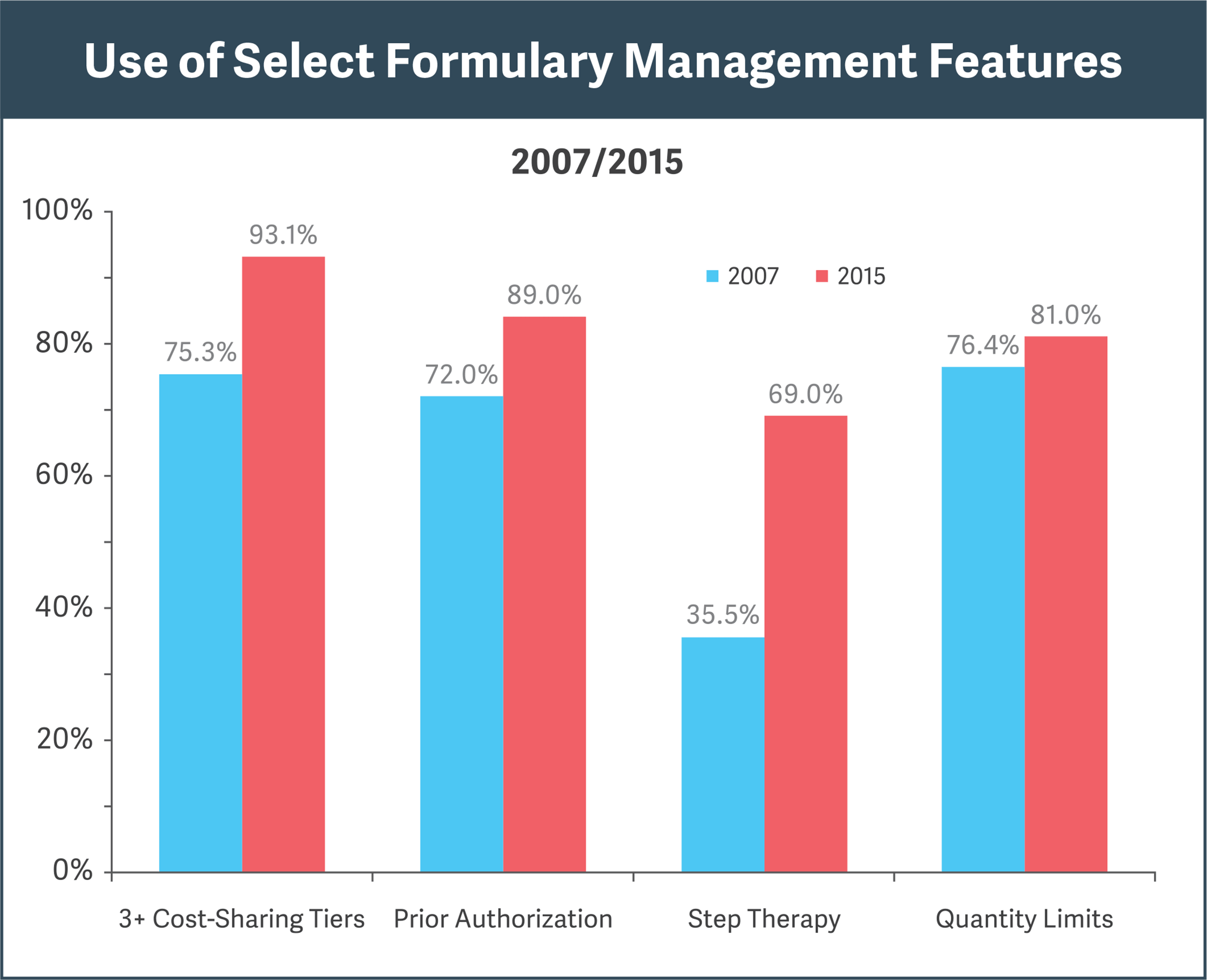
Sources: Pharmacy Benefit Management Institute, “Prescription Drug Benefit Cost and Plan Design Report,” 2015-2016, pp. 18, 31; “Prescription Drug Benefit Cost and Plan Design Report,” 2007, pp. 12, 29
Research shows increasing use of formulary features for cost efficiencies (see figure):
- Cost-sharing “tiers” steer doctors and patients to cheaper alternatives.
- Prior authorization prohibits reimbursement for a drug unless specified conditions are met.
- Step therapy requires that a patient first try and fail on an alternative, less expensive therapy before obtaining reimbursement for the drug in question.
- Quantity limits restrict reimbursement for a drug to specified amounts over a given period.
The use of insurer mechanisms for shaping patients’ and physicians’ prescription drug choices does not guarantee that the connection between prices and quality is perfect and that adjustments to new pharmaceutical developments are instantaneous. But these imperfections are not unique to pharmaceuticals; introducing “new and improved” products is common to many markets.
For these reasons, the pharmaceutical market should not be singled out for failing to make price/quality trade-offs. At a minimum, the insurers’ ability to make tangible connections between prices and quality needs to be considered when evaluating allegations that a product introduction is anticompetitive. ■
Stephen Fink, Managing Principal
Mark J. Lewis, PrincipalAdapted from “The Myth Of ‘Price Disconnects’ In US Pharma Markets,” by Stephen Fink and Mark J. Lewis, published on Law360.com, May 17, 2016.