-
Monitoring Online Signals:
Implications of Internet Data in Pharmacovigilance
The expansion of the Internet has created a variety of new data types related to pharmaceutical drug use and disease areas. Although the implications of this trend for regulators and industry stakeholders are not yet clear, it has become commonplace for Internet users to turn to sites such as Wikipedia in their search for medical and drug information before posting personal information on sites such as Facebook, Twitter, and AskaPatient based on their own experiences.
In recent years, regulatory agencies such as the US Food and Drug Administration (FDA) and the European public-private partnership, Innovative Medicines Initiative, have funded the development of technical frameworks and tools to track and mine social media data for safety signals. While this type of online pharmacovigilance has tended to pinpoint relevant data at the beginning of a drug release and during a major event, it has also triggered concerns from industry stakeholders about potential liability concerns.
Managing Principal Mei Sheng Duh’s ongoing research in collaboration with several Analysis Group colleagues shows that patients are much more likely to report adverse “quality of life” events online than they are to signal serious clinical events, which are more frequently reported by physicians. These online signals of non-threatening side effects have the potential to drown out evidence of more serious adverse events, suggesting that self-reported Internet data may more accurately complement, rather than replace, traditional pharmacovigilance data sources. This has significant implications for industry stakeholders. The continued increase in both the quantity and variety of these social media data raises potential issues in health care litigation as well as in health economics and outcomes research (e.g., patient-reported outcomes), accelerating the need for the development of new analytic approaches.
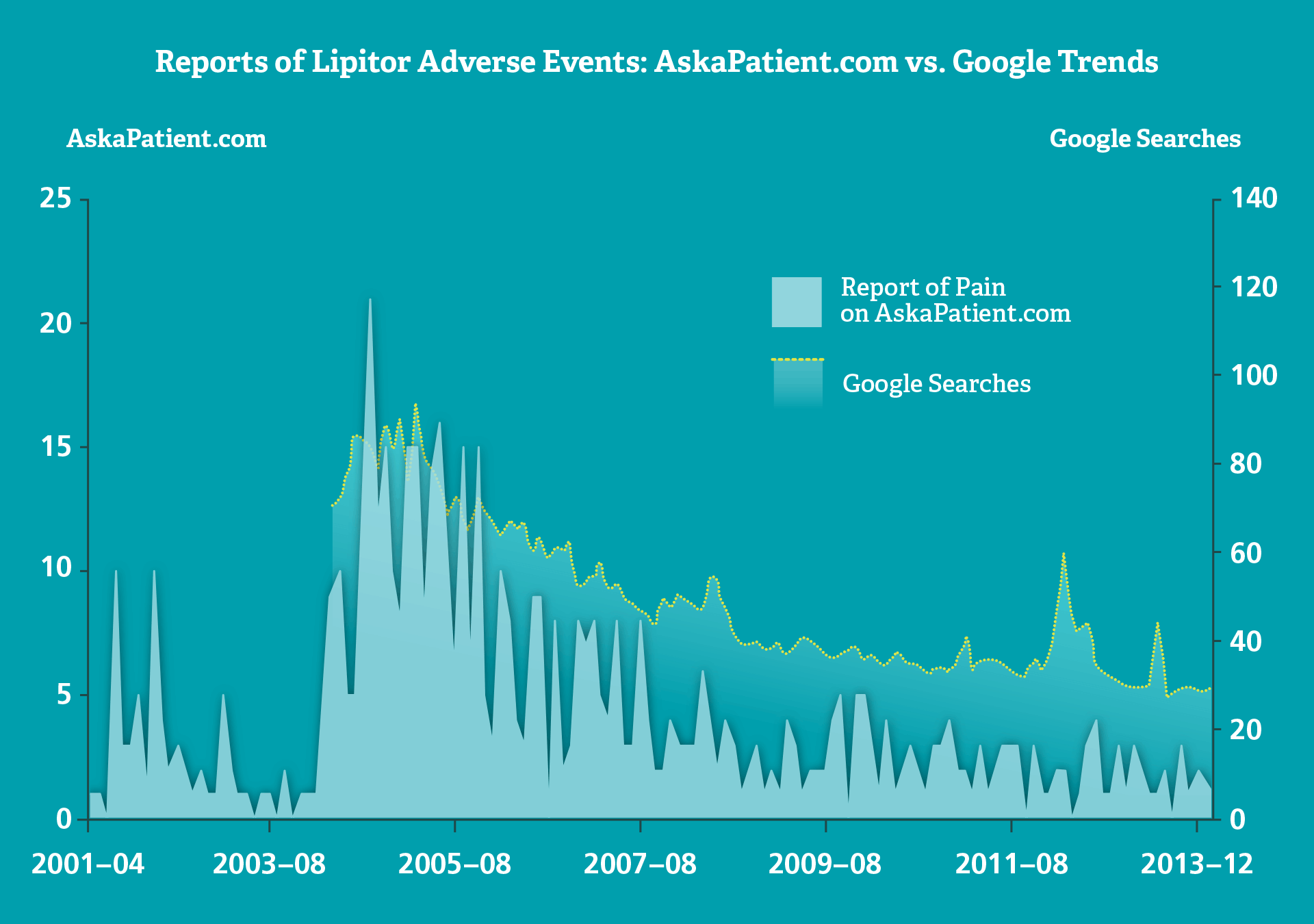
In an assessment of the frequency and pattern of adverse event postings online for Lipitor (atorvastatin), the overall trend across two web sources was quite similar. Reports of pain were highly variable on AskaPatient.com compared with Google searches, with peaks during popular media coverage.
As Dr. Duh notes: “Regulators and litigants are increasingly monitoring social media for reports of suspected adverse events, which have the potential to be handled in the same manner as spontaneous reports.
“But big data on social media is often different,” she adds. “A spike in adverse signals online could coincide with popular media coverage, for example, rather than with an actual increase in adverse events.” ■
From Health Care Bulletin: Fall 2014