Life Cycle of an HEOR Case: Relaxicalm Study
The following is an example of the consulting work our analysts and associates perform as part of a Health Economics and Outcomes Research (HEOR) case team. This illustrative end-to-end example involving a fictitious pharmaceutical company and treatment is intended to inform potential applicants and candidates for the HEOR, Epidemiology & Market Access practice.
At Analysis Group, the consultants in our HEOR practice deliver rigorous, decision-grade evidence and actionable insights to health care stakeholders, including providers, payers, policymakers, and patients. This work spans regulatory submissions, health technology assessment (HTA) submissions, comparative effectiveness analyses, burden-of-illness studies, and patient-reported outcomes research, among others.
The example below provides an illustrative overview of how one type of HEOR study is executed at Analysis Group.
Pharmaceutical company RXCO is developing a new drug, Relaxicalm, to treat anxiety. In parallel with ongoing clinical trials, the company considers what it wants to communicate to stakeholders: There is an unmet medical need for some patients with anxiety, and Relaxicalm can provide safe and effective long-term treatment.
Developing real-word evidence on the burden and trajectory of anxiety among patients can support RXCO’s value proposition and provide context for future interactions with regulatory agencies, physicians, and payers. RXCO issues a request for proposal (RFP) asking Analysis Group for help in designing and executing a real-world study.
After receiving the RFP, Analysis Group begins developing a study aligned with RXCO’s evidence-generation needs. This involves a review of background materials and an assessment of which study designs and data sources could most effectively capture information on unmet medical need in patients with anxiety. This early planning stage involves close collaboration between senior experts at Analysis Group and RXCO to ensure clarity on the strategy, study objectives, and scope of work.
The two agree that the best way to demonstrate the unmet need around anxiety is by analyzing a group of patients’ anxiety levels over a particular period of time. This will involve collaborating with a set of medical centers and collecting and reviewing patients’ anxiety scores using a clinical rating scale found in patients’ medical charts that assesses anxiety severity. Once the study proposal is agreed on by RXCO and Analysis Group, the case work begins.
An HEOR case team is led by a firm partner – a principal or managing principal. The partner works with a manager or vice president to staff team members with skills that are relevant to the case and to support the size and scope of the work. Senior team members provide strategic oversight and methodological guidance, and lead client interactions, while junior team members support day-to-day implementation, including coordination, programming, and documentation. Regular project meetings with the client help maintain momentum, address emerging questions, and ensure ongoing alignment between Analysis Group and RXCO as the study progresses.
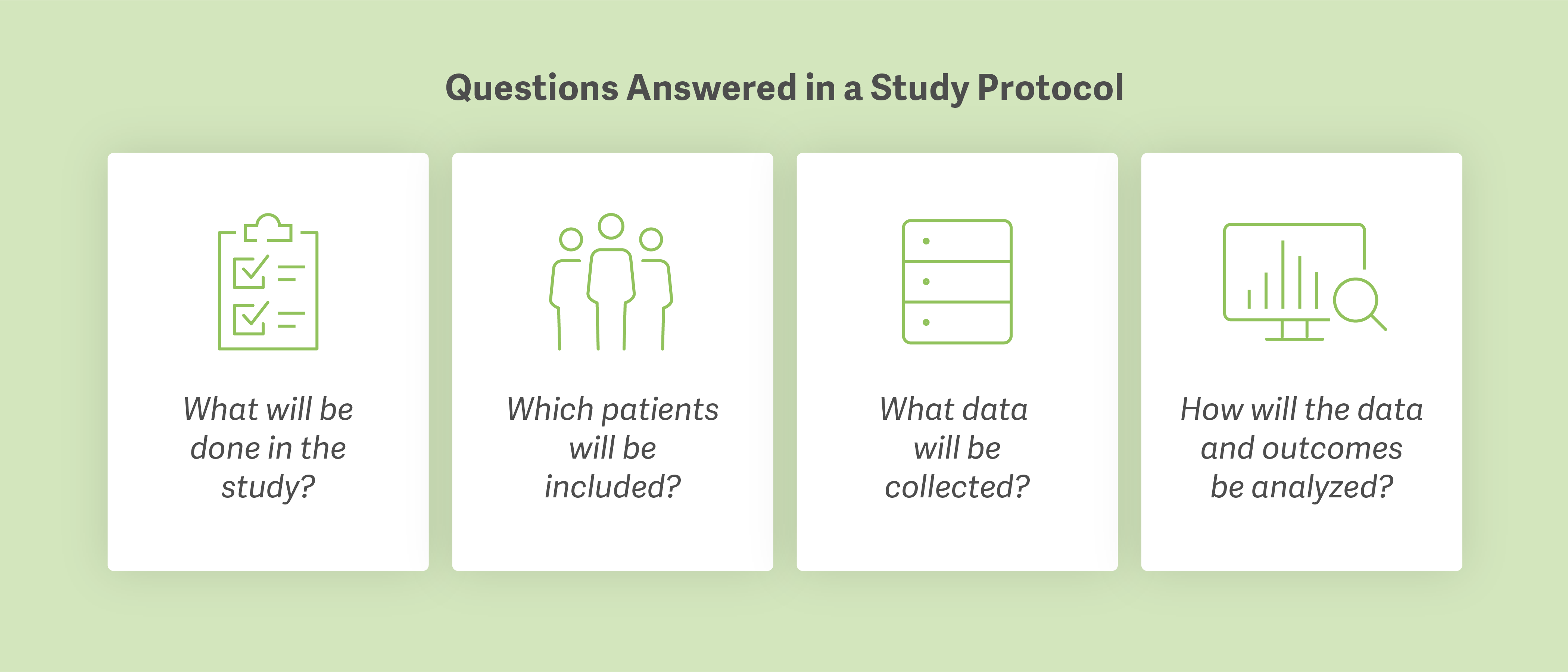
A study proposal builds the framework of an HEOR case. The study protocol serves as the technical foundation. In writing the protocol, the case team outlines the study rationale, study design, variables of interest, which patients will be included in the study population, what data will be collected, and how the team will assess outcomes.
Draft protocols undergo multiple rounds of internal review at Analysis Group to refine the methodological approach and ensure consistency with best practices. The protocol is then shared with RXCO and submitted to the company’s internal protocol review committee, which provides feedback to the firm. After multiple rounds of this review, the protocol is approved and the study is ready for implementation.
At this stage, the team may also partner with a key opinion leader (KOL). In this case, the KOL would likely be a practicing physician or mental health provider associated with the health centers that are participating in the study. The KOL’s role is that of a collaborator and critic: They will consider whether the case team has organized the study in a way that makes sense clinically. They might also suggest other data to collect, or flag errors such as the team unintentionally selecting the most severely anxious patients for the study.
Once the protocol is finalized, the data collection begins. In the Relaxicalm study, the medical centers and participating practitioners conduct the data collection, while the Analysis Group team conducts quality controls such as checking that the values entered make sense. The team will also connect with the chart abstractors – usually external team members such as research assistants at the medical centers – to ask clarifying questions on data as needed. Throughout the process, Analysis Group maintains detailed documentation of all methods, programming steps, and quality-control procedures to ensure transparency, reproducibility, and a clear record of how all results were generated.
Once all of the study data are collected, the HEOR team conducts its analysis. During this stage, the team will be in close communication with RXCO as both sides digest the results and discuss the study’s implications. After completing the pre-specified analyses, the Analysis Group team prepares a comprehensive technical study report for RXCO. This report documents the study design, data sources, analytics methods, results, and interpretation, and supports internal decision making.
Analysis Group and RXCO may pursue publication of the study findings in a peer-reviewed journal. The firm would typically serve as coauthor alongside RXCO’s team members and relevant external experts. Follow-up studies are common depending on newly uncovered research questions – for example, where an especially interesting finding about a particular subgroup might provide a new avenue of investigation.
The study’s findings will have different uses depending on the stakeholder group. Insurance companies could use the information to determine which populations will be covered for Relaxicalm. Providers may use the study’s results to inform treatment decisions. Policymakers might use the study’s findings as broader evidence of the burden of anxiety generally.
Analysis Group’s HEOR consultants typically work on multiple cases or studies at a time. As the Relaxicalm study concludes, the case team members will move on to their other work, and the cycle begins anew.
