Life Cycle of a Health Care Case: Relaxicalm Study
Pharmaceutical products undergo rigorous testing and study prior to market entry, and much of this work takes place outside the familiar framework of clinical trials. The studies, data collection, and analyses behind new treatments are crucial parts of health care research and medical innovation.
At Analysis Group, the consultants in our Health Economics & Outcomes Research (HEOR) practice deliver evidence and actionable insights to health care stakeholders, including providers, payers, policymakers, and ultimately – though indirectly – to patients. This work includes burden-of-illness research, comparative effectiveness research, the study of patient-reported outcomes, clinical research and study design, personalized medicine, and rare disease and orphan drug research.
Let’s take a closer look at the work behind an HEOR case at Analysis Group.
Pharmaceutical company RXCO is developing a new drug, Relaxicalm, to treat anxiety. Before Relaxicalm can be put on the market, it must undergo rigorous testing. As RXCO prepares for Relaxicalm’s clinical trials, the company considers what it wants to communicate to stakeholders: There is an unmet need for some patients with anxiety, and Relaxicalm can provide safe and effective long-term treatment. But a clinical trial isn’t always the right framework for communicating the properties of a therapy. That’s because they have limitations:
- Clinical trials are conducted in very controlled environments. A trial group may not be representative of the general population living with anxiety.
- Clinical trials are expensive and time constrained. The study period might be too short to analyze a drug’s long-term effectiveness.
- Clinical trials don’t tell the whole story around standard of care. Unanswered questions could include: What is the landscape of current products available for treating anxiety? How are patients using the products already on the market, and what’s the user percentage breakdown?
RXCO determines that more information to support Relaxicalm is needed beyond clinical trial evidence, so it approaches Analysis Group for help in planning a study. The two agree that the best way to demonstrate the unmet need around anxiety is by analyzing a group of patients’ anxiety levels over a particular period of time. This will involve collaborating with a set of medical centers and collecting and reviewing patients’ anxiety scores using a clinical rating scale found in patients’ medical charts that assesses anxiety severity. The participating medical centers are often selected through existing partnerships with Analysis Group or by contracting with key experts or principal investigators who have previously worked with the firm. Once the study proposal is agreed upon between RXCO and Analysis Group, the case work begins.
An HEOR case team is led by a firm partner – a principal or managing principal. The partner works with an Analysis Group staffing coordinator, as well as a manager or vice president, to source team members with skills that are relevant to the case and to support the size and scope of the work. For Relaxicalm, this means experience in databases and statistics. However, if an analyst or associate has previously raised their hand and expressed interest in mental health, or cases involving themes such as burden of illness, they may also be asked to join the team.
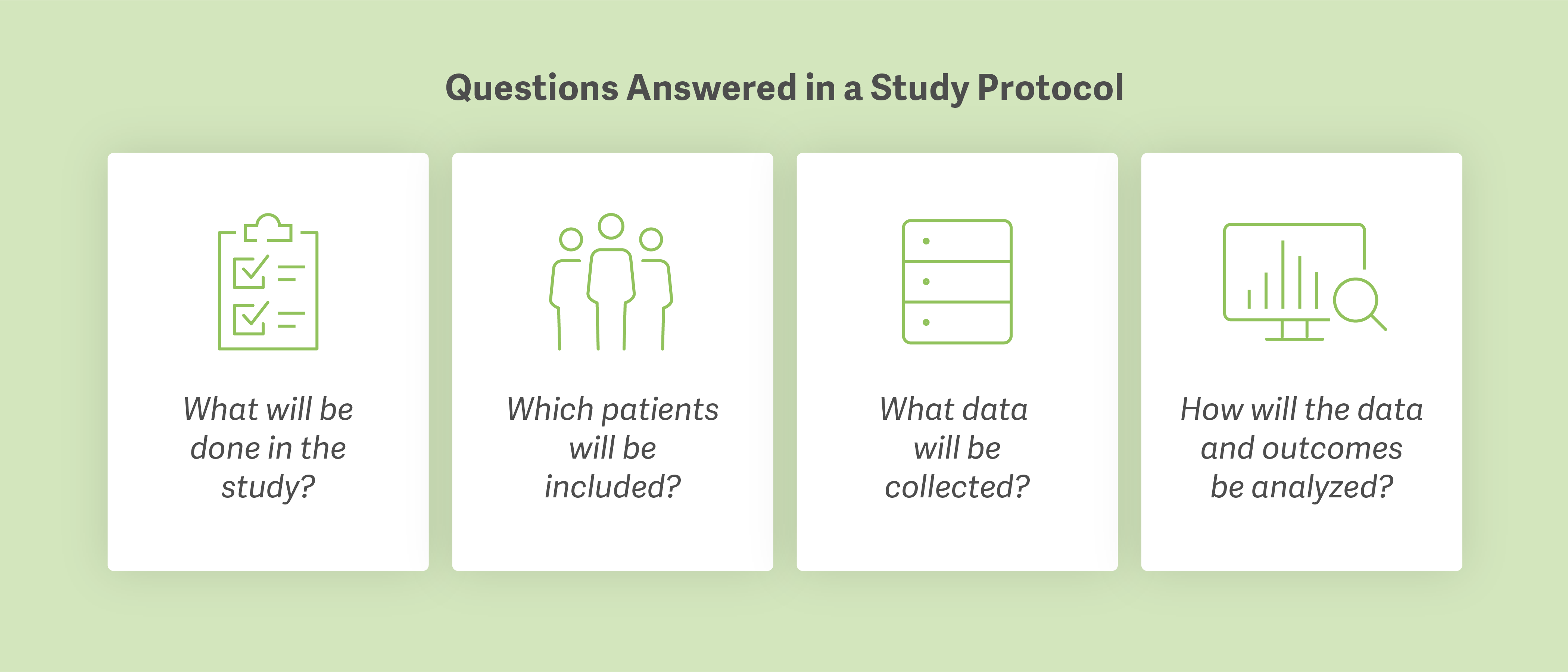
A study proposal builds the framework of an HEOR case. The study protocol lays out the details. Some clients have their own template to work from, but, in RXCO’s case, this is handled by the Analysis Group team. In writing the protocol, the case team determines what will be done in the study, which patients will be included in the study population, what data will be collected, and how the team will analyze data and assess outcomes. A protocol is particularly important because it allows RXCO to demonstrate – with an appropriate level of rigor – that the research meets the standard for peer-reviewed publication.
At this stage, the team may also partner with a key opinion leader (KOL). In this case, the KOL would likely be a practicing physician or mental health provider associated with the health centers that are participating in the study. The KOL’s role is that of a collaborator and critic: They will consider whether the case team has organized the study in a way that makes sense clinically. They might also suggest other data to collect, or flag errors such as the team unintentionally selecting the most severely anxious patients for the study.
Once the protocol is finalized and the KOL has weighed in, the data collection begins. In the Relaxicalm study, the medical centers and participating practitioners conduct the actual chart review and data collection, while the Analysis Group team conducts quality controls such as checking subsets of the data to ensure the team has the information it needs for the study. The team will also question the chart abstractors – usually external team members such as research assistants at the medical centers – on whether additional details from the data are needed, or to provide interim sets of results on partial data as it is collected.
Once all of the study data is collected, the HEOR team conducts its analysis. During this stage, the team will be in close communication with RXCO as both sides digest the results and discuss the study’s implications. This information is then turned into a study report, which is usually written by the case team or a medical writer. A study report is typically for the client’s internal use only, while an abstract or poster about the study might be presented later at a conference.
The study’s findings will have different uses depending on the stakeholder group. Insurance companies can use the information to determine reimbursement rates and which populations will be covered for Relaxicalm. Providers will use the study’s results to inform treatment decisions. Policymakers may use the study’s findings as broader evidence of the burden of anxiety generally. Follow-up studies are common and usually case-by-case depending on newly uncovered research questions – for example, where an especially interesting finding about a particular subgroup might provide a new avenue of investigation for RXCO and/or Analysis Group.
Analysis Group’s HEOR consultants typically work on multiple cases or studies at a time. As the Relaxicalm study draws to a close, the case team members will move on to their other work, and the cycle begins anew.
